Explain the Stability of Different Conformation of N Butane
Isomerism Isomers are non-identical compounds with the same molecular formula. This is visualized at 180 dergrees on the graph and is the most stable conformation.

Most Stable Conformation Of N Butane Is Youtube
There are now three rotating carbon-carbon bonds to consider but we will focus on the middle bond between C 2 and C 3Below are two representations of butane in a conformation which puts the two CH 3 groups C 1 and C 4 in the eclipsed position.
. Here the compound in option A is in anti staggered form. 1 3-dimethylcyclo hexane has two chiral centers and can have four stereoiso mers 2 2 4. Now let us consider butane a slightly larger molecule.
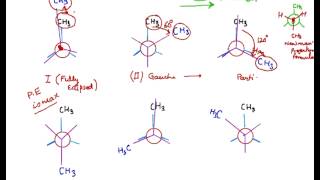
Gauche-staggered I fully eclipsed IIeclipsed III and -staggered IV. Generally Butane has four conformation isomers which are fully eclipsed gauche eclipsed and anti butane conformational isomers. CONFORMATION OF BUTANEN-Butane is a four carbon alkane derived from ethane in which one hydrogen atom on each carbon is substituted by a methyl group.
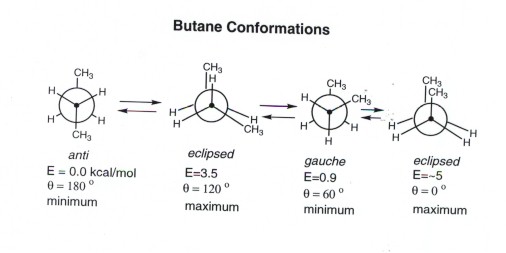
This is the highest energy conformation for butane due to. Conformational analysis is the study of the energetics between different rotamers and is useful for understanding the stability of different isomers by taking into account the spatial orientation and through-space interactions of substituents. Butane Conformational Energy Diagram.
Structural constitutional isomers Stereoisomers 3. The butane molecule is drawn in Chem 3D and energy minimized. Topic- conformational analysis of n- BUTANE Made by- Sophia Mubashir 1.
This molecule can be found in several forms known as isomers. Conformations of n-butane are as under Staggered conformation has minimum repulsion so it is the most stable. They are hydrocarbons because these compounds are composed only of C and H atoms.
If different substituents are present all will be chiral. Eclipsed conformation occurs in a conformation when hydrogen atoms are attached to two carbons areas nearest to each other as possible. There are two energy minima the gauche and anti forms which are both staggered and thus have no torsional strain.
Correct option is A Anti staggered conformation is more stable than gauche and eclipsed conformations. Arrange the following conformations of n-butane according to their increasing stability. Normally when we rotate the molecule of butane at the axis of the C-C bond it shows different conformation isomerism.
We started with the staggered conformation of butane right here which has a certain potential energy and we went from this staggered conformation to this eclipsed conformation right here by rotating 60. The third conformation is anti conformation which is the most stable of all as the heavier methyl groups are situated opposite each other at a dihedral angle 180. The higher energy of eclipsed bonds is known as eclipsing strain.
Isobutane is a structural isomer of butane. To explain further two substituents X and Y on adjacent atoms A and B are in the closest proximity indicating that the torsion angle XABY is 0. Staggered conformations about carbon-carbon single bonds are more stable have a lower potential energy than the corresponding eclipsed conformations.
When the dihedral angle 60 or 300 gauche conformation in butane the methyl groups are farther apart and therefore the potential energy drops by 41 kcalmol. The anti staggered conformation of n-butane is more stable than gauche staggered and eclipsed conformations of n-butane. This places the molecule at its optimal energy which corresponds to the staggered conformation where the two methyl groups are furthest from each other.
Both butane and isobutane have the same chemical. Conformational analysis can be used to predict and explain products selectivity mechanisms and. Butane is an organic compound.
Types The two main classes of isomers are called structural isomers and stereoisomers. The molecule will normally occupy a steady state one of low energy and experience a change to another steady state only upon absorbing enough energy to reach and pass through the uneven intervening conformation. Draw Newman and sawhorse projections for the eclipsed and staggered conformations of ethane.
The order of stability is staggered gauche eclipsed energy order eclipsed gauche staggered. And these pictures are just stills from the actual video. The anti form is the absolute energy minimum since the gauche form has a small steric interaction between the two methyl groups.
When we look at the chemical structure of butane we can see that it has two. It is the most stable. As in anti conformation the bulky groups are oppositely placed at an angle of 180 0 thereby reducing the steric hindrance between the groups resulting in the stability of the molecule.
In anti staggered n-butane the methyl groups are placed at a dihedral angle of 1 8 0 0 and the steric hindrance is minimal in anti-form than in gauche form. If the forces are strong different conformations vary greatly in energy or stability. At a dihedral angle of 60 degrees one hydrogen of each of the.
When the dihedral angle 0 or 360 syn conformation in butane the two methyl groups are in close proximity with the molecule therefore the potential energy is at its highest. It is the most unstable conformation because the two groups are present at a dihedral angle of 0 which increases the steric strain in the compound. Lets us discuss these isomers below.
Both butane and isobutane are gaseous hydrocarbon compounds. Heres an energy diagram showing the different conformations we saw in the video. In butane the gauche-conformer is less stable than the anti-conformer by about 09 kcalmol.
Trans isomer has a twofold rotational axis hence it is also achiral. Actually there are only three the cis-1 3-dimethylcyclohexane has a plane of symmetry and is achiral. Stereochemistry of Butane.
Conformation Of Butane N Butane Conformation Of N Butane Youtube

The Order Of Stability For The Conformations Of N Butane Among These Is Anti I Gauche Youtube
No comments for "Explain the Stability of Different Conformation of N Butane"
Post a Comment